Delta H And Delta S Chart
Formation reactions youre only allowed to have one mole of a product.
Delta h and delta s chart. You just need to isolate Delta S in that equation. Gibbs Free Energy Change and Spontaneity. The units for Delta S are quite complicated.
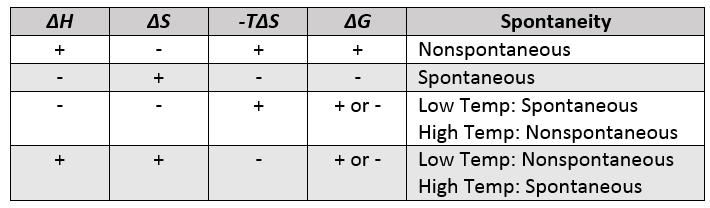
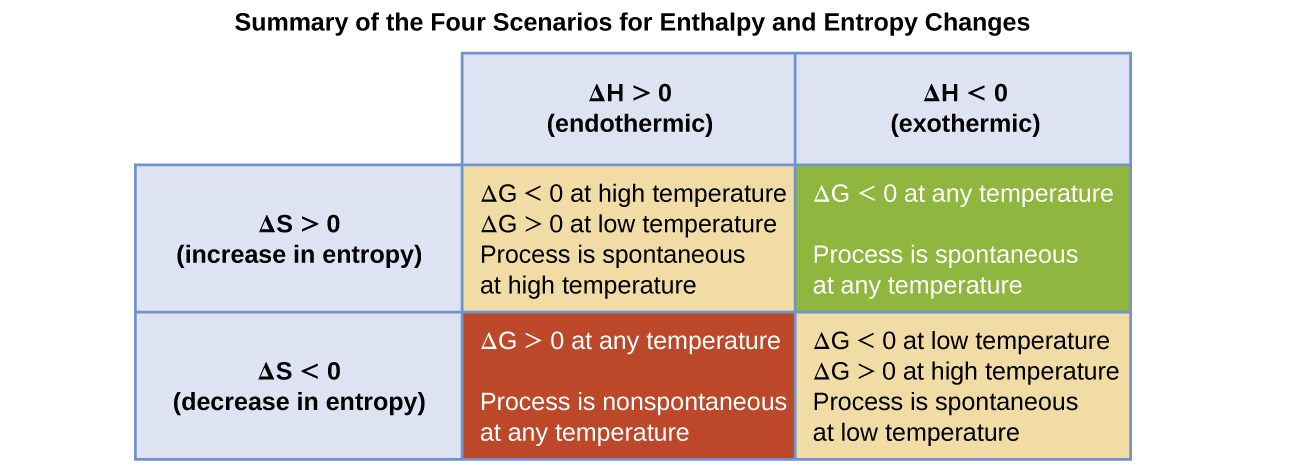
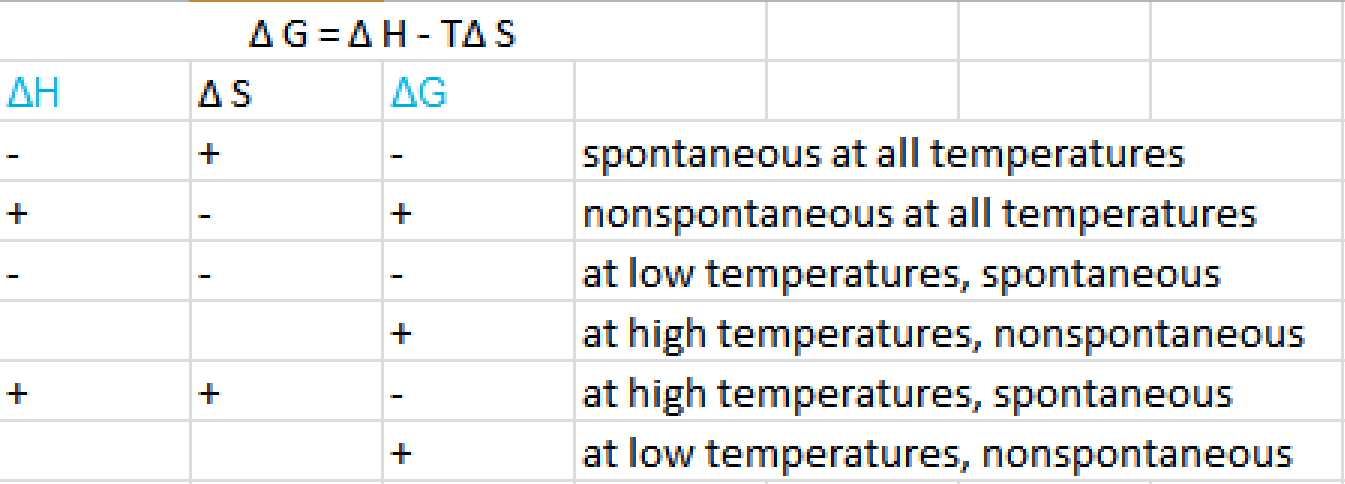
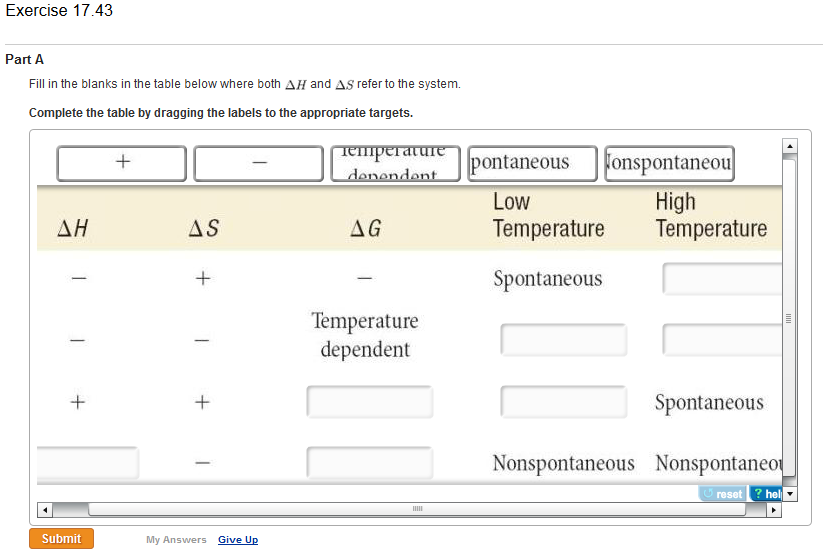
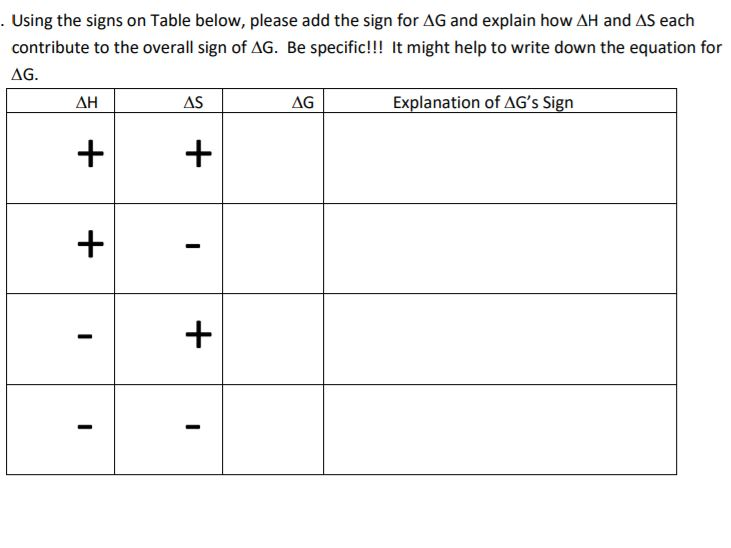
Δ G can be negative under the following conditions. Lets look at this process. Delta S is a term used to denote the total change in entropy.
Determine Delta S for the reaction using Standard Molar Entropies and Hess Law of Summation. Δ G Δ H T Δ S. This measured value is used to calculate the frac tional value of delta E which evolves from the hue difference between two color samples alone.
Formula State H f 0 S0 G f 0 AlBH 4 3 ℓ 1632 28911 14477 AlBH 4 3 g 1255 37907 14644 AlCH 3 3 g 7406 000 000 AlCH 3 3 ℓ 13640 20941 1004 AlNO 3 36H 2O s 285048 46777 220388 AlNO 3 39H 2O s 375706 56902 292964 AlOAc 3 s 189242 000 000 AlOH 3 s 128449 7113 130583 Al3 aq 53137 32175 48534 Al 2CH 3 6. The absolute color difference between two sample s is known as the hue difference delta h. Our delta H equals -826.
According to Gibbs- Helmholtz equation. Delta h delta e p delta v The enthalpy internal energy and volume are all changed but the pressure remains the same. Al 2 SiO 5 andalusite-259027-244266.
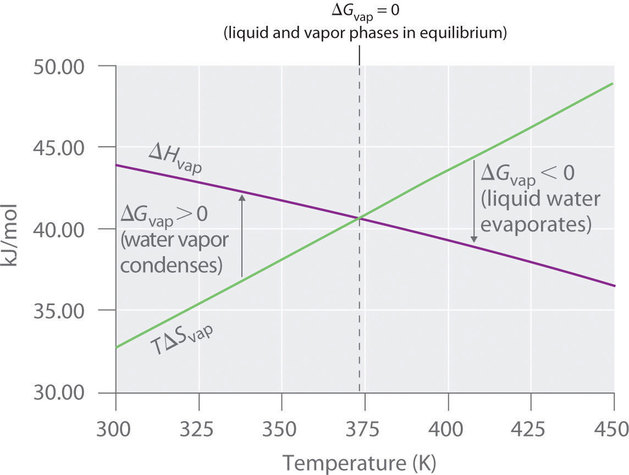
Therefore if the entropy of the system increases after a certain event the value of delta S will be positive. For reaction to be spontaneous Δ G should be negative Δ G 0. Umair Iftikhar June 23 2021.
Δ Hf of CNs 101 kJmol do not forget to divide by the 3 coefficient Posted in Chemistry. 3 CO 2 4 H 2 O burning propane gives off heat and makes more molecules. 183 Gibbs Free Energy Delta G Delta H T Delta S 184 Delta G Delta H Delta S and Formation Reactions 185 Gibbs Free Energy and the Equilibrium Constant.
DeltaGDeltaH-TDeltaS In order to calculate the free energy for a reaction the above equation should be used. Thus the temperature of the experiment in Kelvin must be known. On the other hand if the entropy of a system.
Specific heat C P JK. Delta h cp delta T. Both Δ H and T Δ S are negative.
These values are valid for the Temperature 25 C. C 3 H 8 5 O 2. Enthalpy change says a lot about whether a chemical reaction is positive or negative.
Substance form Enthalpy Δ f H kJ. Flying four legs instead of three allows four separate calculations of wind speed direction to confirm stable winds at that test airspeed. You use several formulas to calculate the enthalpy change almost universally used delta H equation is H cmT.
Chem Table Gibbs Free Energy of Formation Delta G May 2nd 2010 Author. So this actually is a formation reaction well not really because it doesnt have one mole. Al 2 SiO 5 kyanite-259429-244388.
Once you do that using basic algebra youll see that. Use the Δ H and balanced chemical equation below and calculate the Δ Hf of CNs. Delta S Delta G Delta H -T.
Δ H is negative and T Δ S is positive. Gibbs Δ f G kJ. The last chart of this Section shows how each knot of accumulated Delta V_c uncertainty affects the Delta H_c uncertainty at various altitudes and temperatures.
From our derivation of the enthalpy equation the change of specific enthalpy is equal to the heat transfer for a constant pressure process. Iron and oxygen combine together to make rust. Delta H 1-1105 1000 - 1-220 Delta H -1105 - -220 1105 kJ.
Delta S 1 mole1975 Jmole-K 1 mole223 Jmole-K - 1 mole2837 Jmole-K Delta S 4205. Entropy is a measure of the degree of randomness or the degree of disorder in a given system. Differences in brightness are ignored during the calculation of delta H.
Table of Thermodynamic Values - UWMadison. G H TS a H is negative and S is positive then both contribute to making G negative for example. Delta H is the change in enthalpy during a chemical reaction which may possibly be positive or negative.
2 HCNg AgCNs - Ags H2 g 3 CNsΔ H -113 kJmol. What is Delta S and Delta H. But this it does have a delta H and Im going to.
This is an exothermic process. Since Delta G Delta H TDelta S all you need to do is rearrange the equation if youre trying to figure out how to calculate Delta S. B H is negative and S is also negative but T is small enough for example.
Also DeltaS is given in J K-1 mol-1 thus it must be converted into kJ K-1 mol-1 otherwise the DeltaG value will be incorrect.